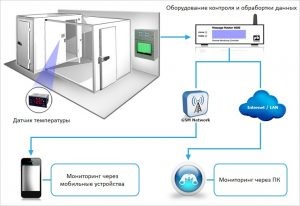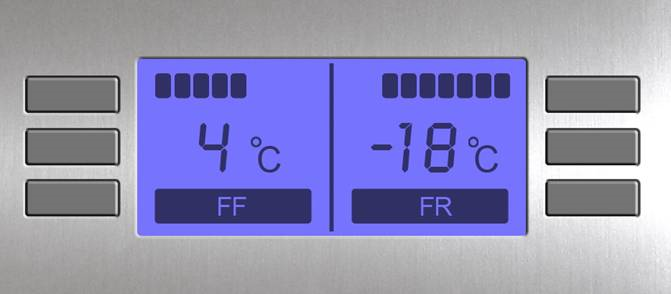
Temperature monitoring in cold chambers and main requirements for storage facilities according to GDP:
-
Medicinal products should be stored apart from other goods under the conditions specified by the manufacturer to avoid any deterioration by light, moisture, or incorrect temperature;
-
Temperature and humidity should be regularly monitored and recorded. Temperature and humidity monitoring records should be regularly reviewed;
-
Where products require controlled-temperature storage conditions, storage facilities should be provided with temperature recording devices or other instruments recording temperature excursions.
Deliveries to customers
Medicinal products should be transported in such a way that they are secure and not subjected to unacceptable degrees of heat, cold, light, moisture, or other adverse influence, nor to attack by microorganisms or pests (insects and animals).
Helps maintain the integrity and quality of medicinal products under the controlled environment.
Temperature monitoring

It helps maintain the integrity and quality of medicinal products under controlled environmental conditions. It is to be understood that medicinal products should be transported under similar conditions by appropriately specialized means (refrigerated trucks).
In practice, the temperature can be attributed to critical parameters of refrigeration equipment. And what about humidity? Is it necessary to conduct humidity monitoring? It should be noted that to control humidity, a cold chamber should be provided with appropriate instruments ensuring such possibility, which is expensive, and in most cases not justified. Additionally, products stored in cold rooms and refrigerators are most often packed in a tight (leak-free) primary package and respectively protected against excessive moisture.
Temperature monitoring: small volume refrigerators
-
A device recording minimum and maximum temperatures (device recording temperature excursions) should be at least installed. Minimum and maximum temperatures should be recorded daily; the device should be reset to zero after data reading. The accuracy must be ±0,5°С. The device display should be positioned outside the refrigerator to read the data without opening the door (additional risks for products). In case of a power failure, the battery should ensure continued monitoring for at least 48 hours.
-
The installation of alarm systems (notification in case of temperature excursions outside upper and lower limits) is preferable. It should be noted that alarms must be activated even in the event of brief excursions outside the acceptable limits.
-
The probe should be placed within the load to record the load rather than the air temperature, which can be quickly increased by opening the door. The products should not be placed near the coolant as the temperature may fall below the accepted minimum, as well as near the compressor as the temperature may exceed the accepted maximum. Auto-defrost should not affect the temperature within the load.
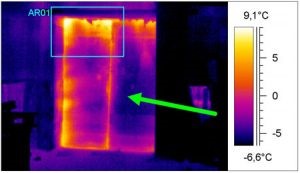
Temperature monitoring: large volumes (in excess of 6 m3)
-
These units should be monitored with suitable electronic temperature-recording devices. Records should be printed and checked daily. The battery should ensure the operation of the refrigerator for at least 48 hours in the event of a power failure. Portable data loggers may be used for monitoring.
-
An alarm (notification in case of temperature excursions outside upper and lower limits) should be fitted and suitable limits set. Opening the door, changes in temperature and loading/unloading operations should not trigger a false alarm. Alarm functions should be tested regularly. It should be remembered that an alarm is of little use if there is nobody to hear and respond to it, which will lead to the loss of product quality!
-
The recorder probe should be placed within the load. When temperature distribution indicates that there are high-risk areas where temperature excursions higher or lower than the accepted range are expected, an additional device has to be installed in this area.
-
The auto-defrost facility should not affect the temperature within the load.
-
Recording probes should preferably be independent of controlling probes!
Temperature monitoring: cold chambers
-
It is recommended to use several monitoring devices. If only one data logger is used, it should be located at the point that reflects the temperature in the whole unit. This point must be supported by validation studies. It is recommended to place the device recording maximum and minimum temperatures in the event of monitoring system failure. The device display should be positioned outside the unit.
-
For cold chambers, it is less important to place probes within the load than for refrigerators. The temperature variations caused by opening the door must be minimal provided that it is not left open for prolonged periods.
-
As a rule, goods sensitive to temperatures greater than 8°C should not be stored next to the door, and goods susceptible to temperatures below 2°C should not be placed in the airflow from the refrigeration unit.
-
Recording probes should preferably be independent of controlling probes!
Sensor for cold chambers
Sensor for cold chambers is a measuring instrument intended to generate measurement signals in the form suitable for their further transformation, processing, and/or storage, which cannot be perceptible to the observer.

Refrigeration Equipment Monitoring
Refrigeration Equipment Monitoring is the process of continuous monitoring of process parameters to avoid or at least reduce the probability of failure of components of a refrigerating unit and ensure its safe operation.