
Cleanroom design is the process of developing design solutions for rooms that meet GMP cleanliness level requirements (A, B, C, and D).
Cleanrooms
Cleanrooms are widely used in healthcare and pharmaceutical industries and electronic, aerospace, chemical, and food industries. Cleanrooms are defined as environmentally controlled enclosed spaces in which airborne particulates and microorganisms are kept within specified limits. The main objective of the cleanroom is the protection of the product and environment from contamination, protection of personnel, protection from dangerous vapors and microorganisms, etc. In the medical field, cleanrooms are primarily surgical units, intensive care units, and intensive therapy units.
Cleanroom enclosing structures are intended to create a barrier to prevent contamination coming into the cleanroom from outside and vice versa. These enclosing structures include insulated materials, ventilation systems, filtration elements, and access control systems.
Cleanroom design. Stages
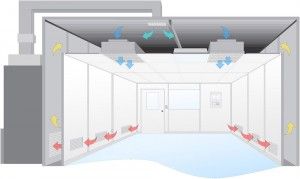
First of all, cleanroom design means the development of conceptual design, which must pass an expert evaluation (design qualification).
Conceptual design is the first stage of the design, a fundamental basis of any project.
Design qualification is a documented verification that the proposed design (sterile product manufacture) meets the requirements of the User Requirements Specification (URS) and GMP.
Cleanroom design is carried out based on the conceptual design that has passed qualification - design and working documentation are developed.
